Uses Of Ni Dmg 2
| Physical Chemistry Virtual Lab Physical chemistry (also called physicochemistry) is the explanation of macroscopic, microscopic, atomic, subatomic, and particulate phenomena in chemical systems in terms of physical concepts; sometimes using the principles, practices and concepts of physics like thermodynamics, quantum chemistry, statistical mechanics and dynamics. Spectrophotometry Cryoscopy Ebullioscopy EMF measurement Determination of Viscosity of Organic Solvents Adsorption Isotherm Verification of Tafel Equation Determination of Viscosity Average Molecular Weight of Polymer Calorimetry -Water equivalent Calorimetry Calorimetry -Heat of Neutralization |
| Organic Chemistry Virtual Lab Organic chemistry is a discipline within chemistry which involves the scientific study of the structure, properties, composition, reactions, and preparation (by synthesis or by other means) of chemical compounds that contain carbon. Detection of Functional Groups Detection of Elements: Lassaigne鈥檚 Test Separation of Compounds Using Column Chromatography Purification by Fractional distillation/crystallisation Purification by Steam distillation/crystallisation Laser Flash Photometer Organic Preparations - Allylation of Isatin Estimation of Aspirin Estimation Of Glucose Calculation of 位max of Organic Compounds Using Woodward Fieser Rules |
| Inorganic Chemistry Virtual Lab Inorganic chemistry is the branch of chemistry concerned with the properties and behavior of inorganic compounds. This field covers all chemical compounds except the myriad organic compounds (carbon based compounds, usually containing C-H bonds). Water analysis-Determination of Physical parameters Water analysis-Determination of Chemical parameters Acid Base Titration Gravimetric Estimation of Barium Gravimetric Estimation of Nickel Crystal Field Theory Group Theory Alloy Analysis (Brass) Soil Analysis-Determination of Specific conductivity of Soil Soil Analysis-Determination of pH of Soil |
| Advanced Analytical Chemistry Virtual Lab Analytical chemistry is the branch of chemistry concerned with studying the properties of materials and development of tools used to analyze materials. It is the science of sampling, defining, isolating , concentrating and preserving samples. Soil Analysis-Determination of Available Organic Carbon content in the Soil Soil Analysis-Determination of Available Nitrogen content in the Soil by Kjeldahl method Soil Analysis-Determination of Available Phosphorus content in the Soil by Bray's method Electrogravimetric Estimation of Metals Estimation of Phosphate Content in Soft Drinks Flame Photometry Polarography - Determination of Unknown Concentration of Cadmium Polarography - Determination of Unknown Concentration of Vitamin C |
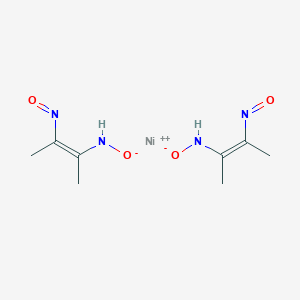
The Gravimetric Estimation of Nickel: The nickel is precipitated as nickel dimethyl glyoxime by adding alcoholic solution of dimethyl glyoxime C 4 H 6 (NOH) 2 and then adding a slight excess of aqueous ammonia solution. When the pH is buffered in the range of 5 to 9, the formation of the red chelate occurs quantitatively in a solution. Ni(dmg) 2 2+ complex (Figure 1d) Dimethylgloxine (dmg) is a bidentate ligand that chelates a large number of metals. Only two dmg molecules are required per metal center because Ni(dmg) 2 2+ has a square-planar geometry. Add 1% dmg to the aqueous complex. A solid pink/red precipitate forms, the insoluble Ni(dmg) 2 2+ complex. Nickel compounds are considered cancer suspect agents. Chemicals and Solutions: 0.1M Nickel sulfate, NiSO4. Ammonium hydroxide, NH4OH. 25% Ethylene diamine in ethanol. 1% Dimethylglyoxime in ethanol. Materials: stirring rods. 5 hydrometer cylinders. Convert dmg to iso download. Stirring rod. Beakers or crystallizing dishes.
| Names | |
|---|---|
| IUPAC name | |
| Other names N,N-Dimethylglycine | |
| Identifiers | |
| |
| 3DMet | |
| 1700261 | |
| ChEBI | |
| ChemSpider |
|
| DrugBank | |
| ECHA InfoCard | 100.012.971 |
| EC Number |
|
| 82215 | |
| KEGG |
|
| MeSH | dimethylglycine |
| RTECS number | |
CompTox Dashboard(EPA) | |
| |
| |
| Properties | |
| C4H9NO2 | |
| Molar mass | 103.121 g·mol−1 |
| Appearance | White crystals |
| Odor | Odourless |
| Density | 1.069 g/mL |
| Melting point | 178 to 182 °C (352 to 360 °F; 451 to 455 K) |
| Boiling point | 175.2 °C (347.4 °F; 448.3 K) |
| Hazards | |
| GHS pictograms | |
| GHS Signal word | Warning |
| H302 | |
| Lethal dose or concentration (LD, LC): | |
| >650 mg kg−1(oral, rat) | |
| Related compounds | |
Related alkanoic acids | |
| Dimethylacetamide | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| verify (what is ?) | |
| Infobox references | |
Dimethylglycine (DMG) is a derivative of the amino acidglycine with the structural formula (CH3)2NCH2COOH. It can be found in beans and liver. It can be formed from trimethylglycine upon the loss of one of its methyl groups. It is also a byproduct of the metabolism of choline.
When DMG was first discovered, it was referred to as Vitamin B16, but, unlike true B vitamins, deficiency of DMG in the diet does not lead to any ill-effects and it is synthesized by the human body in the citric acid (or Krebs) cycle meaning it does not meet the definition of a vitamin.
Uses[edit]
Dimethylglycine has been suggested for use as an athletic performance enhancer, immunostimulant, and a treatment for autism, epilepsy, or mitochondrial disease.[2] There is no evidence that dimethylglycine is effective for treating mitochondrial disease.[3] Published studies on the subject have shown little to no difference between DMG treatment and placebo in autism spectrum disorders.[4][5]

Uses Of Ni Dmg 2017
Biological activity[edit]
Dimethylglycine has been found to act as an agonist of the glycine site of the NMDA receptor.[6]
Preparation[edit]
This compound is commercially available as the free form amino acid, and as the hydrochloride salt [2491-06-7 ]. DMG may be prepared by the alkylation of glycine via the Eschweiler–Clarke reaction. In this reaction, glycine is treated with aqueous formaldehyde in formic acid that serves as both solvent and reductant. Hydrochloric acid is added thereafter to give the hydrochloride salt. The free amino acid may have been obtained by neutralization of the acid salt, which has been performed with silver oxide.[7]
- H2NCH2COOH + 2 CH2O + 2 HCOOH → (CH3)2NCH2COOH + 2 CO2 + 2 H2O
References[edit]
- ^'dimethylglycine - Compound Summary'. PubChem Compound. USA: National Center for Biotechnology Information. 16 September 2004. Identification. Retrieved 24 April 2012.
- ^'Dimethylglycine'. About Herbs, Botanicals & Other Products. Memorial Sloan–Kettering Cancer Center. December 8, 2009.
- ^Pfeffer, Gerald; Majamaa, Kari; Turnbull, Douglass M.; Thorburn, David; Chinnery, Patrick F. (2012-04-18). 'Treatment for mitochondrial disorders'. The Cochrane Database of Systematic Reviews (4): CD004426. doi:10.1002/14651858.CD004426.pub3. ISSN1469-493X. PMID22513923.
- ^Bolman WM, Richmond JA (June 1999). 'A double-blind, placebo-controlled, crossover pilot trial of low-dose dimethylglycine in patients with autistic disorder'. Journal of Autism and Developmental Disorders. 29 (3): 191–4. doi:10.1023/A:1023023820671. PMID10425581.
- ^Kern JK, Miller VS, Cauller PL, Kendall PR, Mehta PJ, Dodd M (March 2001). 'Effectiveness of N,N-dimethylglycine in autism and pervasive developmental disorder'. Journal of Child Neurology. 16 (3): 169–73. doi:10.1177/088307380101600303. PMID11305684.
- ^Lin, Jen-Cheng; Chan, Ming-Huan; Lee, Mei-Yi; Chen, Yi-Chyan; Chen, Hwei-Hsien (2016). 'N,N-dimethylglycine differentially modulates psychotomimetic and antidepressant-like effects of ketamine in mice'. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 71: 7–13. doi:10.1016/j.pnpbp.2016.06.002. ISSN0278-5846. PMID27296677.
- ^Clarke, H. T.; Gillespie, H. B.; Weisshaus, S. Z. (1933). 'The Action of Formaldehyde on Amines and Amino Acids'. Journal of the American Chemical Society. 55 (11): 4571. doi:10.1021/ja01338a041.